Trailblazers: Andrew Zydney Drives Biotech Innovation

When Andrew Zydney was tapped to be the inaugural director of Penn State’s Center of Excellence in Industrial Biotechnology in 2018, he recognized the unique opportunity to leverage his research expertise in downstream bioprocessing, which refers to the purification of biological molecules used to treat various diseases and medical disorders.
“Anything that makes those processes more efficient has the potential to have an enormous impact on the delivery of life-saving therapeutics to patients,” he said. “These issues become even more significant in less developed parts of the world. We can argue that drug costs are too high in the United States but the reality is that drugs that cost tens of thousand dollars per patient per year are inaccessible to almost all of the population of Africa, Southeast Asia, and places like that. We must do something to lower the costs of those molecules to make them more widely available to patients who need them.”
The high cost of prescription drugs plays a significant role in the debate over healthcare and is often cited as a major barrier to treatment. You might think that the most expensive part of manufacturing a new drug is the creation of the complex molecules themselves, but up to 80 percent of the cost lies in purification. Injected drugs need to be incredibly pure; they bypass all the ways our body normally protects us from foreign molecules. These biological molecules are also fragile, making sterilization a major challenge—membrane filtration is currently used to ensure the sterility of essentially all biotherapeutics, ranging from insulin to monoclonal antibodies. Membranes work at room temperature and don’t require the addition of toxic chemicals.
Zydney’s research group actively collaborates with many of the leading biopharmaceutical companies that commercialize new biotherapeutics, including Pfizer, Merck, Bristol-Myers Squibb, and Eli Lilly. Six of the top-selling drugs are monoclonal antibodies; these are primarily anticancer agents or treat immunologic disorders like psoriatic arthritis and Crohn’s disease. “These collaborations enable us to work on molecules that would be impractical or far too expensive to make ourselves, and we get to tap into the knowledge base and practical experience that our industrial collaborators have. They also ensure that our research really targets the most important challenges that need to be addressed in the production of these biological products,” he explained.
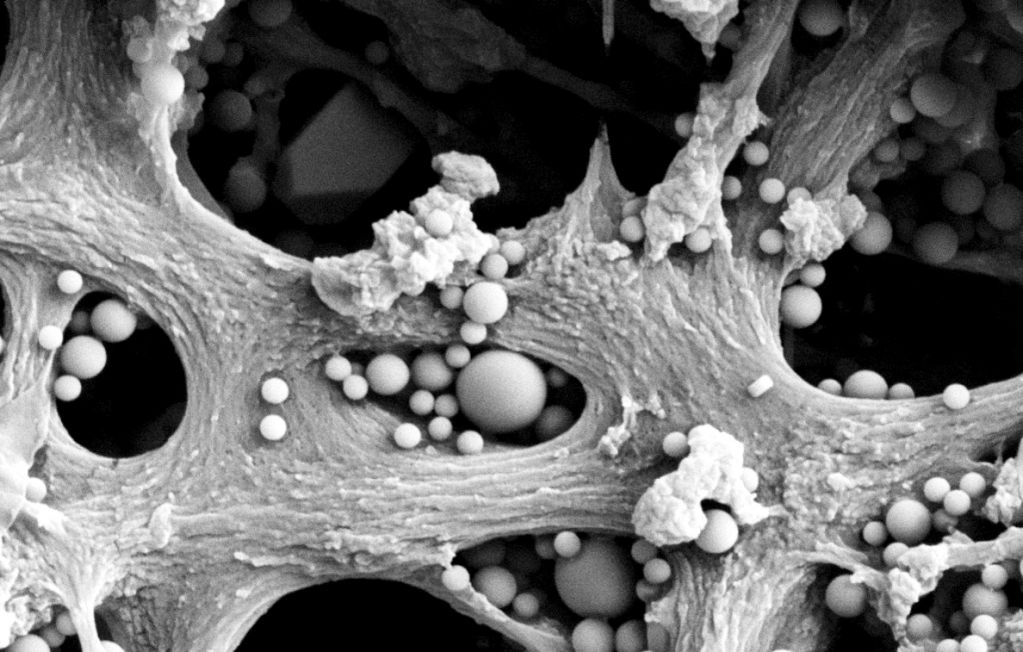
His recent project with Pfizer involved designing membrane systems to purify a glycoconjugate, which is a complex that incorporates a polysaccharide from the outer coat of an infectious bacteria as the basis for a vaccine. Polysaccharides are a polymer of different sugars; they are not alive and cannot reproduce. The bacterial polysaccharides are chemically coupled to a very immunogenic protein that stimulates the immune system. It turns out there one of the major problems in using membranes to purify these glycoconjugates is membrane fouling due to the “stickiness” of this glycoconjugate.
“We learned a lot about the fundamentals of how polysaccharides interact with and ultimately pass through the nanometer-size pores of these membranes,” Zydney said. “We've been able to develop novel strategies for using membranes to purify these biomolecular complexes, which has assisted Pfizer in the development of their purification processes for these kinds of products.”
Zydney headed the Department of Chemical Engineering for 10 years, and until recently was the editor-in-chief of the Journal of Membrane Science. He is currently an associate editor of Biotechnology and Bioengineering, one of the leading bioprocessing journals. Last year, he was selected to receive the Alan S. Michaels Award in the Recovery of Biological Products from the Biotechnology Division of the American Chemical Society.

When he’s not conducting membrane research, Zydney leads the Center of Excellence in Industrial Biotechnology, which serves as a hub for students, faculty, and industry. The unit’s work includes research and educational initiatives focused on biopharmaceutical manufacturing, production of bio-based chemicals and fuels, and food biotechnology. It was established with a $4.92 million gift from CSL Behring that also provided funds to acquire state-of-the-art equipment for the CSL Behring Fermentation Facility, a major core facility that is an engine for collaboration, innovation, biological training, and research.
“The fact that the Center cuts across education, research and outreach in a very significant way, with workforce development being emphasized more than specific research deliverables, is quite unusual,” said Zydney. “We specifically focus on undergraduate involvement, including opportunities to tap into funding to help support projects and paid internships in the Fermentation Facility. Students can also find leadership roles within the Society of Industrial Biotechnology, which is directly supported by the Center.”
The Center also provides funding through CSL Behring scholarships, undergraduate summer research scholarships, and seed grants, and is currently seeking proposals for seed grant funding for interdisciplinary research, curricular development, or educational activities in the broad area of industrial biotechnology. The last round of seed grants funded projects that ranged from protein purification to biofuels and involved faculty in four different departments spanning three different academic colleges.
To Zydney, industrial biotechnology is by nature transdisciplinary, as it draws from cutting-edge research in the life sciences, whether recombinant DNA technology, sequencing human and plant genomes or the development of genetically modified organisms. “Whether you are creating bio-derived chemicals or new pharmaceuticals, it requires collaboration from people who both understand the basic science and can carry it all the way through to the solution of industrially and societally relevant problems,” said Zydney.
He used a seed grant to fund a project in the area of CRISPR technology, a new approach for editing genes that is predicted to be the next wave of therapeutic strategies. Patients with hemophilia, for instance, are currently treated with daily or weekly injections of a clotting factor to prevent bleeding problems.
“A better approach, one might argue, would be to fix their genes so they have the correct gene sequence to make the desired clotting factor. If we can properly edit their genome, they would be cured,” Zydney said. “CRISPR technology has the potential to do exactly that using a bacterial enzyme that can cut DNA using a piece of guide RNA to target a specific gene among the 20 thousand or so genes in the human genome.”
Zydney focused on one step in the process of building a therapeutic: developing a cost-effective purification protocol using membrane technology. CRISPR Cas9 protein and guide RNA are assembled into a complex, but excess RNA that doesn’t bind with Cas9 has to be separated.
“My group tries whenever possible to get ahead of the curve and say, "Okay, this is the next generation biotherapeutic. They're going to need to purify this molecule. What can we do to identify strategies that will make it easier to develop cost-effective purification strategies for this type of novel therapeutic?" That's what the goal of the seed grant was,” Zydney said.
Zydney first had to produce the Cas9 for subsequent experimentation. That work was done at the Fermentation Facility using genetically engineered bacteria.
“It was critical that we demonstrate to an external funding agency that we have the ability to produce sufficient quantities of Cas9 for our research efforts. The fact that we had both the Fermentation Facility and experts in fermentation technology made it a success. We fully expected to be able to do this, but the seed grant provided the funds to demonstrate the technology, enabling my students to do some runs that didn't work, figure out why they didn't work, and then improve the process,” Zydney said.
“The concept is simple. The complex between the Cas9 and the guide RNA is bigger than the guide RNA alone. In principle, you should be able to get the guide RNA to go through the pores while the complex would be retained by the membrane. However, there are many things that could go wrong. The complex might block the membrane pores. The flexible RNA may become trapped within the depth of the membrane. To the best of our knowledge, nobody has ever tried separating the Cas9 RNA complex from the residual guide RNA using a membrane system,” he said.
Zydney’s work on protein purification will continue after the grant has ended, and he hopes to prepare a proposal for the National Science Foundation this summer.
“The fact that we both have the capabilities at Penn State through the fermentation facility, and that we were given the seed funding to demonstrate that we are able to produce the Cas9 protein, is incredibly valuable,” he explained. “That type of money is very hard to come by. People want research results; they don't want to support the background learning needed to set up the basic experimental procedures that will be needed to conduct the proposed research.”
“Most of the seed funding, a large majority of the dollars, was used to get us to the point where we could do the membrane separation experiments that are the core of our research on the purification of these novel therapeutics.”